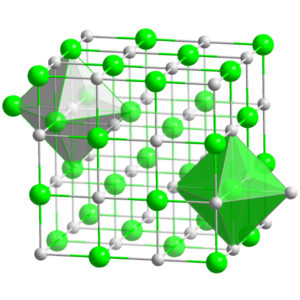
The properties of Magnesium
The important interaction between phosphate and magnesium ions makes magnesium essential to the basic nucleic acid chemistry of all cells of all known living organisms. More than 300 enzymes require magnesium ions for their catalytic action, including all enzymes using or synthesizing ATP and those that use other nucleotides to synthesize DNA and RNA. The ATP molecule is normally found in a chelate with a magnesium ion.
Numerous pharmaceutical preparations of magnesium and dietary supplements are available. In two human trials magnesium oxide, one of the most common forms in magnesium dietary supplements because of its high magnesium content per weight, was less bioavailable than magnesium citrate, chloride, lactate or aspartate.
In the UK, the recommended daily values for magnesium are 300 mg for men and 270 mg for women. In the U.S. the Recommended Dietary Allowances (RDAs) are 400 mg for men ages 19–30 and 420 mg for older; for women 310 mg for ages 19–30 and 320 mg for older.
Numerous pharmaceutical preparations of magnesium and dietary supplements are available. In two human trials magnesium oxide, one of the most common forms in magnesium dietary supplements because of its high magnesium content per weight, was less bioavailable than magnesium citrate, chloride, lactate or aspartate.
Magnesium:
- Supports bone health (Daily oral magnesium supplementation suppresses bone turnover in young adult males).
- Promotes heart health (Magnesium and cardiovascular system).
- Mood support (Rapid recovery from major depression using magnesium treatment).
- Promotes sleep quality (The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial).
One study found that men taking 750mg of magnesium per day for four weeks showed a 26% increase in testosterone levels. Magnesium also helps promote quality sleep, which is vital for good testosterone production.