Hojyo Shintaro, Fukada Toshiyuki
Journal of immunology research, 2016
Abstract
Zinc (Zn) is an essential micronutrient for basic cell activities such as cell growth, differentiation, and survival. Zn deficiency depresses both innate and adaptive immune responses. However, the precise physiological mechanisms of the Zn-mediated regulation of the immune system have been largely unclear. Zn homeostasis is tightly controlled by the coordinated activity of Zn transporters and metallothioneins, which regulate the transport, distribution, and storage of Zn. There is growing evidence that Zn behaves like a signaling molecule, facilitating the transduction of a variety of signaling cascades in response to extracellular stimuli. In this review, we highlight the emerging functional roles of Zn and Zn transporters in immunity, focusing on how crosstalk between Zn and immune-related signaling guides the normal development and function of immune cells.
Figures
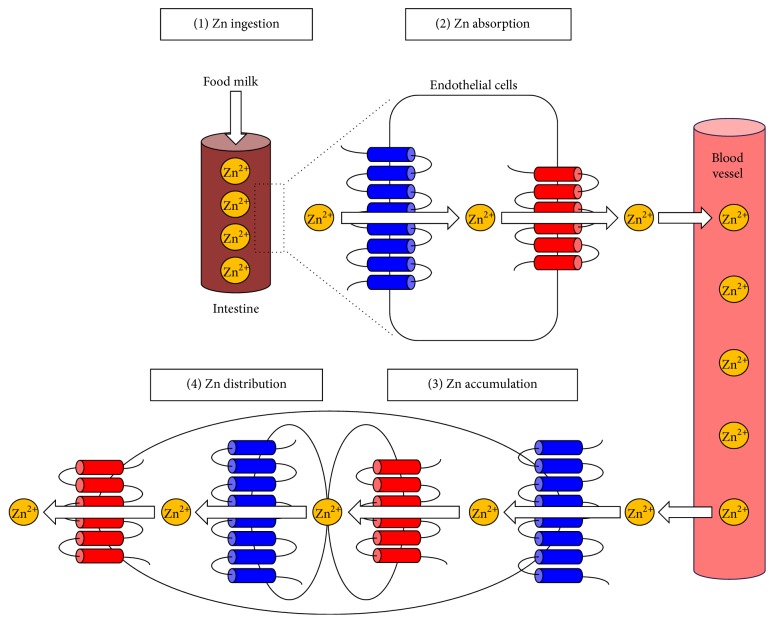 Zn transporters control Zn homeostasis. (1) Zn is ingested from the diet (or from breast milk in infants). (2) Zn is absorbed by intestinal Zn transporters and is released into the bloodstream. (3) Zn is taken up into peripheral cells by Zn transporters located on the plasma membrane. (4) Zn is distributed within the cell by intracellular Zn transporters. Each step is important for maintaining intracellular Zn levels. Disrupting Zn uptake results in ZnD and subsequent pathogenesis. ZIP (blue) and ZnT (red) transporters are indicated.
Zn transporters control Zn homeostasis. (1) Zn is ingested from the diet (or from breast milk in infants). (2) Zn is absorbed by intestinal Zn transporters and is released into the bloodstream. (3) Zn is taken up into peripheral cells by Zn transporters located on the plasma membrane. (4) Zn is distributed within the cell by intracellular Zn transporters. Each step is important for maintaining intracellular Zn levels. Disrupting Zn uptake results in ZnD and subsequent pathogenesis. ZIP (blue) and ZnT (red) transporters are indicated.
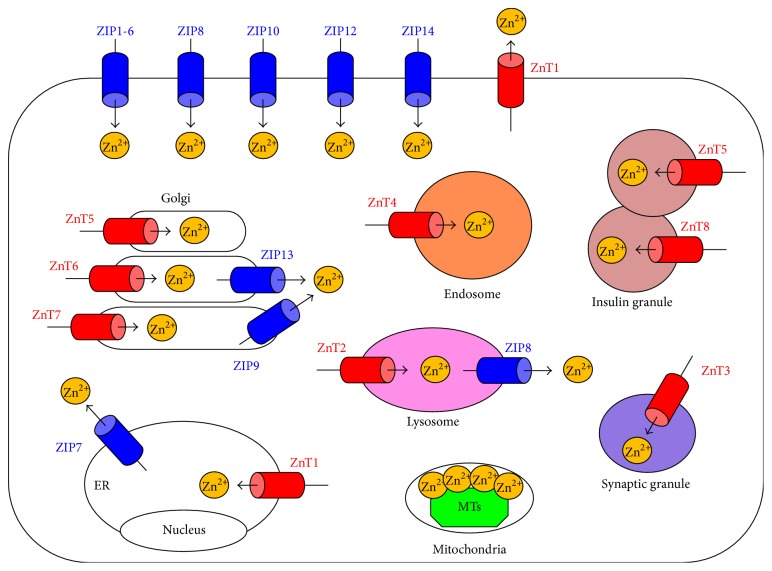 Subcellular localization of Zn transporters and metallothioneins (MTs). The localization of ZIP (blue) and ZnT (red) transporters is determined by the cell type, developmental process, and Zn status. ZIP transporters elevate the intracellular cytoplasmic Zn level by eliciting the influx of Zn from the extracellular space or from intracellular organelles. ZnT transporters reduce the intracellular cytoplasmic Zn level by exporting Zn from the cytosol to the extracellular space or into intracellular organelles or vesicles. MTs (green) contribute to Zn storage. Arrows indicate the predicted direction of Zn mobilization. ER: endoplasmic reticulum.
Subcellular localization of Zn transporters and metallothioneins (MTs). The localization of ZIP (blue) and ZnT (red) transporters is determined by the cell type, developmental process, and Zn status. ZIP transporters elevate the intracellular cytoplasmic Zn level by eliciting the influx of Zn from the extracellular space or from intracellular organelles. ZnT transporters reduce the intracellular cytoplasmic Zn level by exporting Zn from the cytosol to the extracellular space or into intracellular organelles or vesicles. MTs (green) contribute to Zn storage. Arrows indicate the predicted direction of Zn mobilization. ER: endoplasmic reticulum.
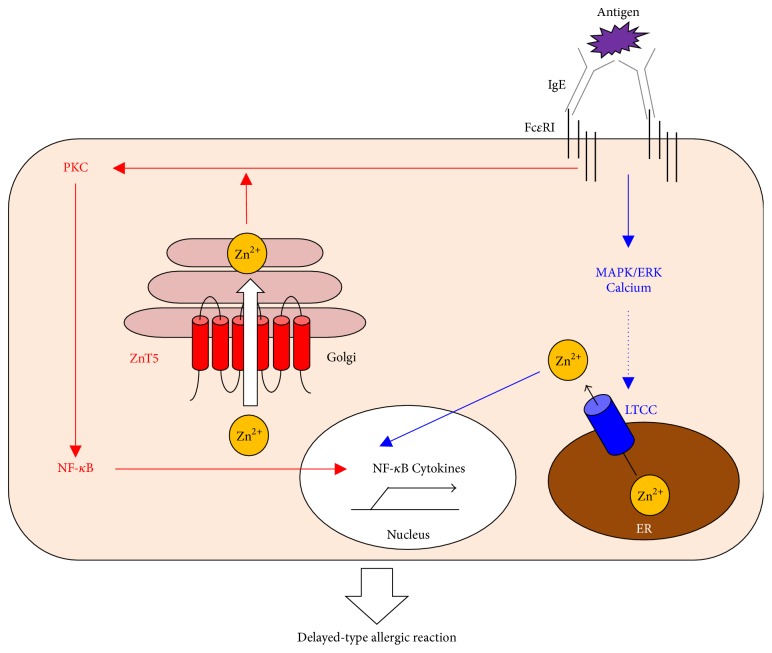 Zn uptake via LTCC and ZnT5 controls the FcεRI-mediated delayed allergic response by mast cells. Zn is indispensable for FcεRI-mediated mast-cell activation. Upon antigen sensitization, LTCC (blue) on the ER membrane acts as a Zn gatekeeper and can rapidly increase the intracellular-free Zn levels in the perinuclear region dependent on calcium and MAPK/ERK signaling (Zn wave). The released Zn then regulates the DNA-binding activity of NF-κB followed by cytokine production. Simultaneously, ZnT5 (red) on the Golgi membrane takes up Zn to regulate the translocation of PKC to the plasma membrane. The resultant NF-κB activation induces inflammatory cytokine production. Thus, these Zn gatekeepers and Zn transporter control mast-cell-mediated, delayed-type allergic reactions.
Zn uptake via LTCC and ZnT5 controls the FcεRI-mediated delayed allergic response by mast cells. Zn is indispensable for FcεRI-mediated mast-cell activation. Upon antigen sensitization, LTCC (blue) on the ER membrane acts as a Zn gatekeeper and can rapidly increase the intracellular-free Zn levels in the perinuclear region dependent on calcium and MAPK/ERK signaling (Zn wave). The released Zn then regulates the DNA-binding activity of NF-κB followed by cytokine production. Simultaneously, ZnT5 (red) on the Golgi membrane takes up Zn to regulate the translocation of PKC to the plasma membrane. The resultant NF-κB activation induces inflammatory cytokine production. Thus, these Zn gatekeepers and Zn transporter control mast-cell-mediated, delayed-type allergic reactions.
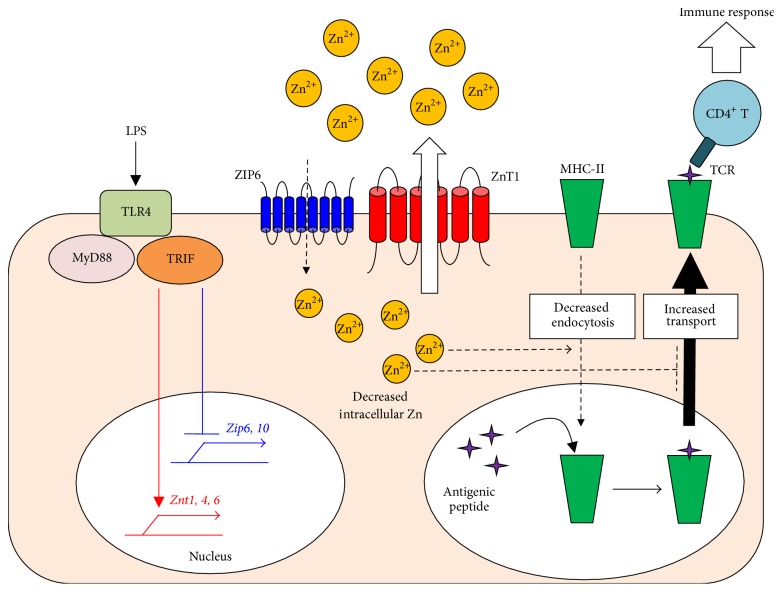 A decrease in intracellular-free Zn is critical for LPS-mediated CD4+ T-cell activation by DCs. LPS, a TLR4 ligand, induces DC activation, which initiates a maturation signal mediated by MyD88 and TRIF. TRIF-mediated signaling reduces the expression of ZIPs (blue) and increases that of ZnTs (red), resulting in a net decrease in the intracellular-free Zn level in DCs. This reduction of intracellular-free Zn is critical for the antigen presentation via MHC-II molecules and the subsequent activation of antigen-specific CD4+ T cells.
A decrease in intracellular-free Zn is critical for LPS-mediated CD4+ T-cell activation by DCs. LPS, a TLR4 ligand, induces DC activation, which initiates a maturation signal mediated by MyD88 and TRIF. TRIF-mediated signaling reduces the expression of ZIPs (blue) and increases that of ZnTs (red), resulting in a net decrease in the intracellular-free Zn level in DCs. This reduction of intracellular-free Zn is critical for the antigen presentation via MHC-II molecules and the subsequent activation of antigen-specific CD4+ T cells.
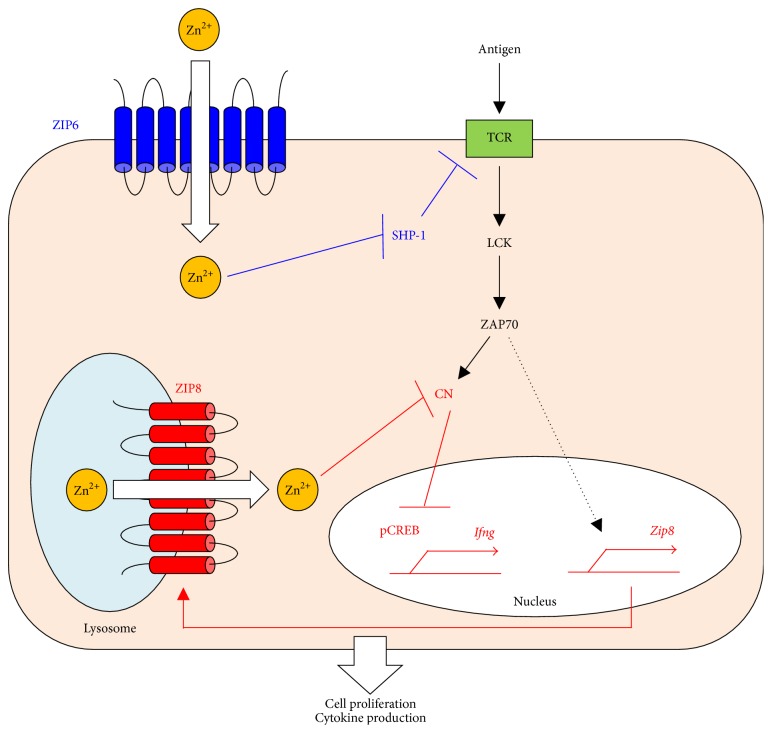 Zn uptake via ZIP6 and ZIP8 potentiates TCR signaling. Through the interaction of DCs and T cells, TCR activation rapidly increases cytoplasmic Zn concentrations, particularly at the subsynaptic compartment, in a manner dependent on ZIP6 (blue). The enhanced influx of Zn reduces SHP-1 recruitment to the TCR activation complex, thereby augmenting ZAP70 activation and leading to a sustained influx of calcium. On the other hand, TCR activation increases ZIP8 expression, which exports Zn out of the lysosome and into the cytoplasm (red). The resultant increase in cytoplasmic Zn inhibits CN, leading to increased CREB activation and the subsequent expression of IFN-γ. Thus, Zn facilitates TCR's functions in proliferation and IFN-γ production.
Zn uptake via ZIP6 and ZIP8 potentiates TCR signaling. Through the interaction of DCs and T cells, TCR activation rapidly increases cytoplasmic Zn concentrations, particularly at the subsynaptic compartment, in a manner dependent on ZIP6 (blue). The enhanced influx of Zn reduces SHP-1 recruitment to the TCR activation complex, thereby augmenting ZAP70 activation and leading to a sustained influx of calcium. On the other hand, TCR activation increases ZIP8 expression, which exports Zn out of the lysosome and into the cytoplasm (red). The resultant increase in cytoplasmic Zn inhibits CN, leading to increased CREB activation and the subsequent expression of IFN-γ. Thus, Zn facilitates TCR's functions in proliferation and IFN-γ production.
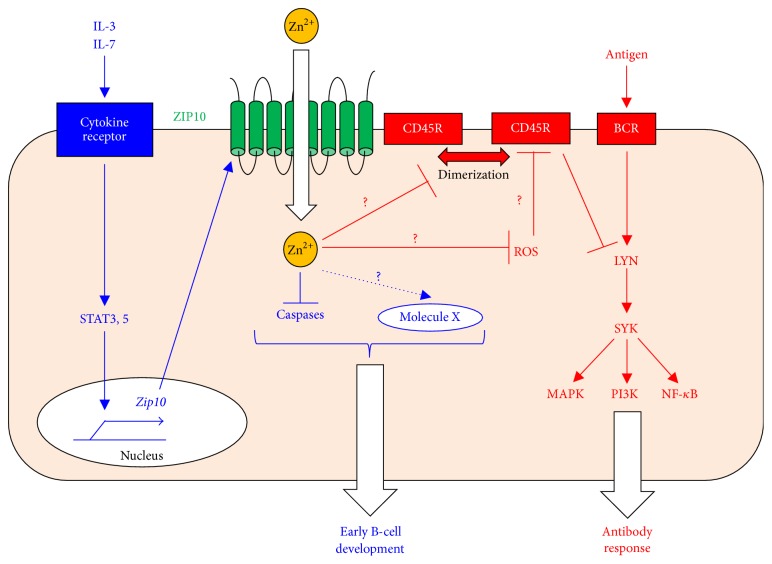 ZIP10's roles in B-cell development and function. In early B-cell development, a cytokine (1st signal) induces JAK-STAT activation (2nd signal), which is converted to an intracellular Zn signal (3rd signal) by ZIP10 upregulation. This system for converting intracellular signals promotes early B-cell survival by inhibiting caspase activation and/or by an unknown mechanism via molecule X (blue). In mature B cells, ZIP10-Zn signaling sets the threshold for the BCR signaling strength by regulating the CD45R PTPase activity (red). Thus, ZIP10 controls antibody-mediated immune responses.
ZIP10's roles in B-cell development and function. In early B-cell development, a cytokine (1st signal) induces JAK-STAT activation (2nd signal), which is converted to an intracellular Zn signal (3rd signal) by ZIP10 upregulation. This system for converting intracellular signals promotes early B-cell survival by inhibiting caspase activation and/or by an unknown mechanism via molecule X (blue). In mature B cells, ZIP10-Zn signaling sets the threshold for the BCR signaling strength by regulating the CD45R PTPase activity (red). Thus, ZIP10 controls antibody-mediated immune responses.
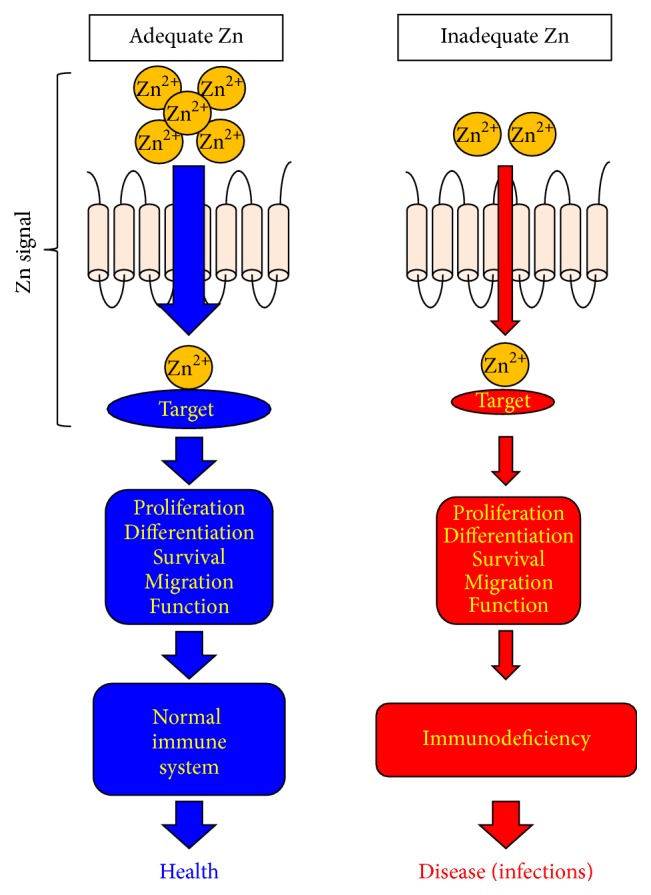 Zn-signal axes in immune system. Each Zn-signal axis targets a specific molecule and controls a variety of cellular activities such as proliferation, differentiation, survival, migration, and function via a distinct signaling pathway to control immune homeostasis and functions. ZnD (red) impairs these Zn-signal axes and leads to disease if there is no redundant machinery.
Zn-signal axes in immune system. Each Zn-signal axis targets a specific molecule and controls a variety of cellular activities such as proliferation, differentiation, survival, migration, and function via a distinct signaling pathway to control immune homeostasis and functions. ZnD (red) impairs these Zn-signal axes and leads to disease if there is no redundant machinery.| PMID: | 27872866 |
|---|---|
| PMCID (Free PMC Article): | PMC5107842 |
| DOI: | 10.1155/2016/6762343 |
| Category: | Immune |
The best supplements with Zinc in Immune category:
- Zinc Picolinate, 100 Tablets (Solgar) - Zinc promotes healthy skin and supports normal taste and vision. It contains among others: Zinc.
- Immune Defence - Zinc Lozenges with Rosehip and Acerola - Your immune system needs daily support to stay in good form, to be able to fight off any pathogens trying to enter your system and to witstand the impact of stress on your body and mind. It contains among others: Zinc.
- L-OptiZinc, 30 mg, 100 Veg Capsules (Now Foods) - L-OptiZinc is a form of Zinc complexed with the amino acids Methionine. It contains among others: Zinc.
- Zinc, 50 mg, 250 Tablets (Now Foods) - Zinc is essential to the normal function of many organs and systems within the body including the skeletal, immune, neurological, and endocrine systems. It contains among others: Zinc.
- Zinc Picolinate, 50 mg, 120 Veg Capsules (Now Foods) - Zinc is essential to the normal function of many organs and systems within the body including the skeletal, immune, neurological, and endocrine systems. It contains among others: Zinc.
Articles similar to "Roles of Zinc Signaling in the Immune System."
- The significance of Zinc for Immune: Zinc as a Gatekeeper of Immune Function. (After the discovery of zinc deficiency in the 1960s, it soon became clear that zinc is essential for the function of the immune system...)
- The role of Zinc in Immune: Zinc Signals and Immunity. (Zinc homeostasis is crucial for an adequate function of the immune system...)
- The significance of Zinc for Immune: Zinc and Immunity: An Essential Interrelation. (The significance of the essential trace element zinc for immune function has been known for several decades...)
- The role of Zinc in Immune: Zinc, Aging, and Immunosenescence: An Overview. (Zinc plays an essential role in many biochemical pathways and participates in several cell functions, including the immune response...)
- The impact of Zinc on Immune: Zinc Signals and Immune Function. (For more than 50 years, it has been known that zinc deficiency compromises immune function...)
- The significance of Zinc for Immune: Zinc: Dietary Intake and Impact of Supplementation on Immune Function in Elderly. (The diet in the elderly does not provide a sufficient level of nutrients needed to maintain an adequate healthy status leading to micronutrient deficiencies and impaired immune response with subsequent development of degenerative diseases...)
- The role of Zinc in Immune: Zinc in Innate and Adaptive Tumor Immunity. (Zinc is important...)
- The impact of Zinc on Immune: Zinc in Human Health: Effect of Zinc on Immune Cells. (Although the essentiality of zinc for plants and animals has been known for many decades, the essentiality of zinc for humans was recognized only 40 years ago in the Middle East...)
- The significance of Zinc for Immune: Immune-enhancing Role of Vitamin C and Zinc and Effect on Clinical Conditions. (Vitamin C concentrations in the plasma and leukocytes rapidly decline during infections and stress...)
- The role of Zinc in Immune: Zinc: Role in Immunity, Oxidative Stress and Chronic Inflammation. (Purpose of review: Zinc is essential for multiple cellular functions including immunity...)
Previous article
Zinc in Innate and Adaptive Tumor Immunity.
Next article
Zinc Transporters and Signaling in Physiology and Pathogenesis.

























































