Fukada Toshiyuki, et al.
JBIC Journal of Biological Inorganic Chemistry, 2011
Abstract
Zinc (Zn) is an essential trace mineral that regulates the expression and activation of biological molecules such as transcription factors, enzymes, adapters, channels, and growth factors, along with their receptors. Zn deficiency or excessive Zn absorption disrupts Zn homeostasis and affects growth, morphogenesis, and immune response, as well as neurosensory and endocrine functions. Zn levels must be adjusted properly to maintain the cellular processes and biological responses necessary for life. Zn transporters regulate Zn levels by controlling Zn influx and efflux between extracellular and intracellular compartments, thus, modulating the Zn concentration and distribution. Although the physiological functions of the Zn transporters remain to be clarified, there is growing evidence that Zn transporters are related to human diseases, and that Zn transporter-mediated Zn ion acts as a signaling factor, called "Zinc signal". Here we describe critical roles of Zn transporters in the body and their contribution at the molecular, biochemical, and genetic levels, and review recently reported disease-related mutations in the Zn transporter genes.
Keywords
Disease; Physiology; Transporter; Zinc; Zinc signaling.
Figures
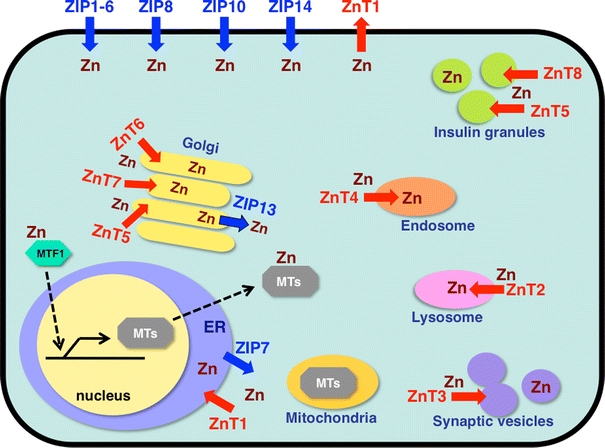 Subcellular localization of zinc (Zn) transporters and metallothioneins (MTs). Localization and potential functions of Zn transporters from the Slc39/Zrt/Irt-like protein (ZIP) (blue) and Slc30/ZnT (red) families, MT, and metal-responsive-element-binding transcription factor 1 (MTF1) within the cell, based on currently available information [, –154]. Arrows show the predicted direction of Zn mobilization. ER endoplasmic reticulum
Subcellular localization of zinc (Zn) transporters and metallothioneins (MTs). Localization and potential functions of Zn transporters from the Slc39/Zrt/Irt-like protein (ZIP) (blue) and Slc30/ZnT (red) families, MT, and metal-responsive-element-binding transcription factor 1 (MTF1) within the cell, based on currently available information [, –154]. Arrows show the predicted direction of Zn mobilization. ER endoplasmic reticulum
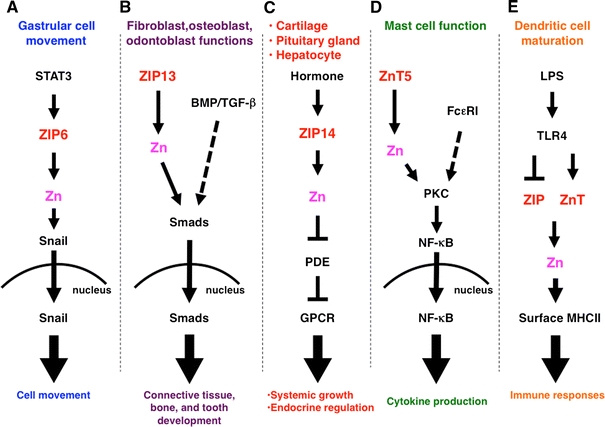 Roles of ZIP and ZnT Zn transporter family members in intracellular signaling. a The signal transducers and activators of transcription 3 (STAT3) downstream target ZIP6 is required for nuclear translocation of the Zn-finger transcription factor Snail, which regulates gastrular cell movement in zebrafish. b ZIP13 is required for the nuclear translocation of Smads in bone morphogenetic protein (BMP)/transforming growth factor beta (TGF-β) signaling, and is involved in tooth, bone, and connective tissue development. c ZIP14, which facilitates G protein-coupled receptor (GPCR) signaling by inhibiting hormone-stimulated phosphodiesterase (PDE) in the pituitary gland, liver, and cartilage, is required for endocrine reactions and systemic growth. d ZnT5 controls protein kinase C (PKC) translocation to the plasma membrane, leading to nuclear factor kappa B (NF-κB)-mediated cytokine production in mast cells under Fc epsilon receptor I (FcεRI) signaling. e Lipopolysaccharide (LPS) stimulation alters the expression of ZIP and ZnT family Zn transporters, resulting in downregulated intracellular Zn levels, followed by dendritic cell maturation and immune responses. TLR Toll-like receptor
Roles of ZIP and ZnT Zn transporter family members in intracellular signaling. a The signal transducers and activators of transcription 3 (STAT3) downstream target ZIP6 is required for nuclear translocation of the Zn-finger transcription factor Snail, which regulates gastrular cell movement in zebrafish. b ZIP13 is required for the nuclear translocation of Smads in bone morphogenetic protein (BMP)/transforming growth factor beta (TGF-β) signaling, and is involved in tooth, bone, and connective tissue development. c ZIP14, which facilitates G protein-coupled receptor (GPCR) signaling by inhibiting hormone-stimulated phosphodiesterase (PDE) in the pituitary gland, liver, and cartilage, is required for endocrine reactions and systemic growth. d ZnT5 controls protein kinase C (PKC) translocation to the plasma membrane, leading to nuclear factor kappa B (NF-κB)-mediated cytokine production in mast cells under Fc epsilon receptor I (FcεRI) signaling. e Lipopolysaccharide (LPS) stimulation alters the expression of ZIP and ZnT family Zn transporters, resulting in downregulated intracellular Zn levels, followed by dendritic cell maturation and immune responses. TLR Toll-like receptor
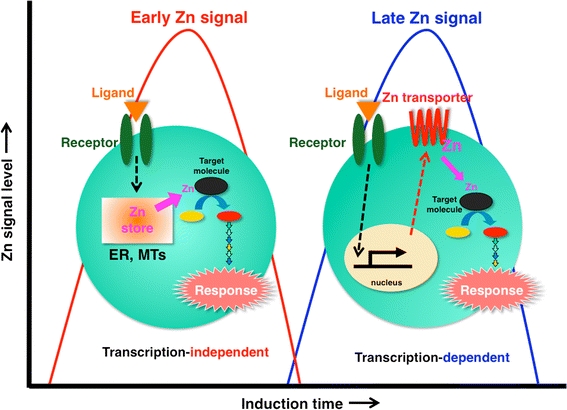 Early and late Zn signaling. Intracellular Zn signaling falls into two types: early Zn signaling (left), in which an extracellular stimulus directly induces elevated Zn levels within several minutes by releasing Zn from a Zn store such as ER or MTs, and late Zn signaling (right), which is induced several hours after stimulation and is dependent on a transcriptional change in Zn transporter expression. Zn waves in mast cells are an example of early Zn signaling
Early and late Zn signaling. Intracellular Zn signaling falls into two types: early Zn signaling (left), in which an extracellular stimulus directly induces elevated Zn levels within several minutes by releasing Zn from a Zn store such as ER or MTs, and late Zn signaling (right), which is induced several hours after stimulation and is dependent on a transcriptional change in Zn transporter expression. Zn waves in mast cells are an example of early Zn signaling
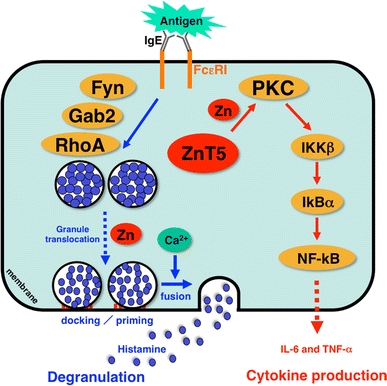 Zn and Zn transporters are indispensable for FcεRI-mediated mast cell activation. Zn is required for multiple steps of FcεRI-induced mast cell activation, including degranulation and cytokine production. Cytosolic Zn regulates the FcεRI-induced granule translocation process, which is mediated by a Fyn/Grb2-associated binder 2 (Gab2)/Ras homologue gene family member A (RhoA)-mediated calcium-independent pathway. Zn and ZnT5 are also required for the translocation of PKC to the plasma membrane and the subsequent nuclear translocation of NF-κB, which leads to the production of cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). IKKβ I-kappa B kinase beta, IkBα I-kappa B alpha
Zn and Zn transporters are indispensable for FcεRI-mediated mast cell activation. Zn is required for multiple steps of FcεRI-induced mast cell activation, including degranulation and cytokine production. Cytosolic Zn regulates the FcεRI-induced granule translocation process, which is mediated by a Fyn/Grb2-associated binder 2 (Gab2)/Ras homologue gene family member A (RhoA)-mediated calcium-independent pathway. Zn and ZnT5 are also required for the translocation of PKC to the plasma membrane and the subsequent nuclear translocation of NF-κB, which leads to the production of cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). IKKβ I-kappa B kinase beta, IkBα I-kappa B alpha
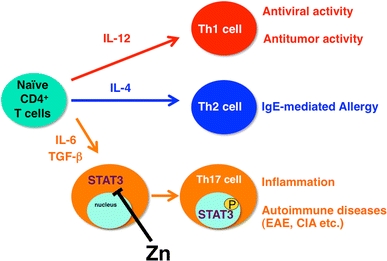 Zn suppresses T helper 17 (Th17) cell development by inhibiting STAT3 activation. Peripheral naive CD4+ T cell precursor cells can differentiate into three subsets of effector T cells (Th1, Th2, and Th17). The differentiation of these subsets is governed by selective cytokines, and each subset accomplishes specialized functions. Th17 cells, which are critical for the development of inflammation and autoimmune disease, are induced by IL-6 and tumor necrosis factor beta (TGF-β). Zn directly binds STAT3, inhibiting its activation by IL-6 and suppressing autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA). P tyrosine phosphorylation
Zn suppresses T helper 17 (Th17) cell development by inhibiting STAT3 activation. Peripheral naive CD4+ T cell precursor cells can differentiate into three subsets of effector T cells (Th1, Th2, and Th17). The differentiation of these subsets is governed by selective cytokines, and each subset accomplishes specialized functions. Th17 cells, which are critical for the development of inflammation and autoimmune disease, are induced by IL-6 and tumor necrosis factor beta (TGF-β). Zn directly binds STAT3, inhibiting its activation by IL-6 and suppressing autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA). P tyrosine phosphorylation
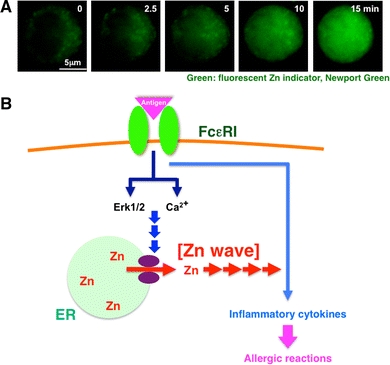 FcεRI increases intracellular free Zn levels, Zn wave: a type of early Zn signaling. a The intracellular Zn level increases within several minutes after antigen stimulation. To monitor the intracellular Zn level, mast cells were treated with the cell-permeable fluorescent Zn indicator Newport Green. Newport Green fluorescence remained steady in the cytoplasm, but gradually increased in the perinuclear and nuclear areas upon FcεRI stimulation. We named this phenomenon a “Zn wave.” b Early Zn signaling in mast cells. Extracellular antigen stimulation induces a Zn wave—a rapid alteration in intracellular Zn level—through the activation of Ca2+ and extracellular-signal-regulated kinase 1 and 2 (Erk1/2) signaling, which might positively affect the signal for inflammatory cytokine-mediated allergic reactions. This suggests that Zn has a role as an intracellular signaling molecule, transducing extracellular stimuli to physiological responses. Violet ovals Zn gatekeepers. (a Modified from Yamasaki et al.
FcεRI increases intracellular free Zn levels, Zn wave: a type of early Zn signaling. a The intracellular Zn level increases within several minutes after antigen stimulation. To monitor the intracellular Zn level, mast cells were treated with the cell-permeable fluorescent Zn indicator Newport Green. Newport Green fluorescence remained steady in the cytoplasm, but gradually increased in the perinuclear and nuclear areas upon FcεRI stimulation. We named this phenomenon a “Zn wave.” b Early Zn signaling in mast cells. Extracellular antigen stimulation induces a Zn wave—a rapid alteration in intracellular Zn level—through the activation of Ca2+ and extracellular-signal-regulated kinase 1 and 2 (Erk1/2) signaling, which might positively affect the signal for inflammatory cytokine-mediated allergic reactions. This suggests that Zn has a role as an intracellular signaling molecule, transducing extracellular stimuli to physiological responses. Violet ovals Zn gatekeepers. (a Modified from Yamasaki et al.
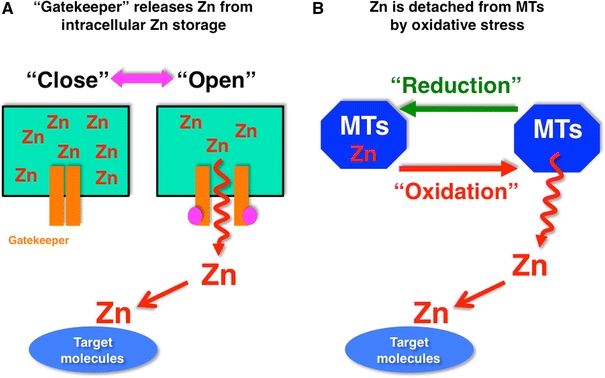 Model for Zn release for the generation of Zn signaling. a Labile Zn in intracellular Zn storage moves through “Zn gatekeepers” into the cytosol, and approaches target molecules. The Zn gatekeepers are closed in the steady state, but are opened by changes in activation status. b Most of the Zn in the cytosol is sequestered in MTs, and is detached from the MTs by intracellular oxidative stress. The rapid increase in intracellular Zn mediated by cellular events may act as an early Zn signaling to change the status of target molecules
Model for Zn release for the generation of Zn signaling. a Labile Zn in intracellular Zn storage moves through “Zn gatekeepers” into the cytosol, and approaches target molecules. The Zn gatekeepers are closed in the steady state, but are opened by changes in activation status. b Most of the Zn in the cytosol is sequestered in MTs, and is detached from the MTs by intracellular oxidative stress. The rapid increase in intracellular Zn mediated by cellular events may act as an early Zn signaling to change the status of target molecules| PMID: | 21660546 |
|---|---|
| PMCID (Free PMC Article): | PMC3176402 |
| DOI: | 10.1007/s00775-011-0797-4 |
| Category: | General properties of Zinc |
Articles similar to "Zinc Homeostasis and Signaling in Health and Diseases: Zinc Signaling."
- The properties of Zinc: Zinc Transporters and Signaling in Physiology and Pathogenesis. (Zinc (Zn) is an essential trace element that is vital in a wide range of cellular machineries because of its effect on the expression and activity of various transcription factors and enzymes...)
- The properties of Zinc: Effect of Zinc Supplementation on Serum Zinc Concentration and T Cell Proliferation in Nursing Home Elderly: A Randomized, Double-Blind, Placebo-Controlled Trial. ( Zinc is essential for the regulation of immune response... Zinc supplementation at 30 mg/d for 3 mo is effective in increasing serum zinc concentrations in nursing home elderly; however, not all zinc-deficient elderly reached adequate concentrations. The increase in serum zinc concentration was associated with the enhancement of T cell function mainly because of an increase in the number of T cells. )
Previous article
Zinc Transporters and Signaling in Physiology and Pathogenesis.
Next article
Zinc: Dietary Intake and Impact of Supplementation on Immune Function in Elderly.

























































