Gammoh Nour Zahi, Rink Lothar
Nutrients, 2017
Abstract
Micronutrient homeostasis is a key factor in maintaining a healthy immune system. Zinc is an essential micronutrient that is involved in the regulation of the innate and adaptive immune responses. The main cause of zinc deficiency is malnutrition. Zinc deficiency leads to cell-mediated immune dysfunctions among other manifestations. Consequently, such dysfunctions lead to a worse outcome in the response towards bacterial infection and sepsis. For instance, zinc is an essential component of the pathogen-eliminating signal transduction pathways leading to neutrophil extracellular traps (NET) formation, as well as inducing cell-mediated immunity over humoral immunity by regulating specific factors of differentiation. Additionally, zinc deficiency plays a role in inflammation, mainly elevating inflammatory response as well as damage to host tissue. Zinc is involved in the modulation of the proinflammatory response by targeting Nuclear Factor Kappa B (NF-κB), a transcription factor that is the master regulator of proinflammatory responses. It is also involved in controlling oxidative stress and regulating inflammatory cytokines. Zinc plays an intricate function during an immune response and its homeostasis is critical for sustaining proper immune function. This review will summarize the latest findings concerning the role of this micronutrient during the course of infections and inflammatory response and how the immune system modulates zinc depending on different stimuli.
Keywords
homeostasis; infection; inflammation; zinc.
Conflict of interest statement
The authors declare no conflict of interest.Figures
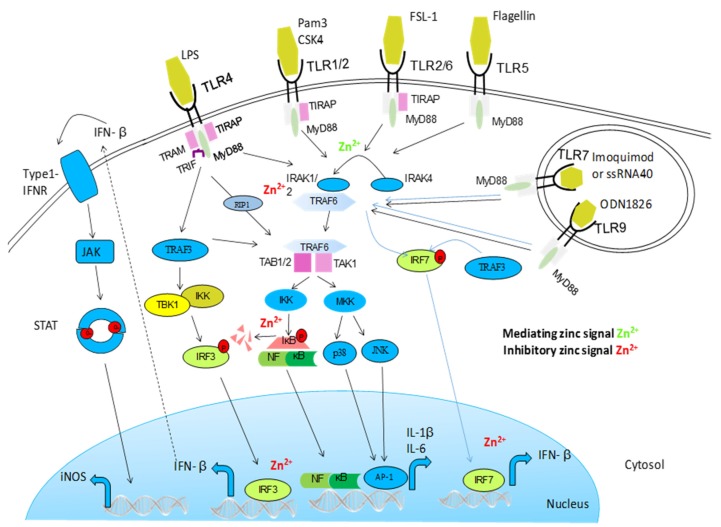 Activation of Toll-like receptor (TLR) signaling pathways is mediated by a complex array of proteins. When TLRs bind ligands, dimerization of the ectodomain of TLRs is induced, bringing their cytoplasmic Toll/IL-1R domains together, resulting in the recruitment of intracellular adapter proteins and initiation of downstream signaling events. Following this, the Toll–IL-1-resistence (TIR) domains of TLRs engage TIR domain-containing adaptor proteins (either myeloid differentiation primary-response protein 88 (MYD88) or TIR domain-containing adaptor protein inducing IFNβ (TRIF) and TRIF-related adaptor molecule (TRAM)). This in turn stimulates downstream signaling pathways that involve interactions between IL-1R-associated kinases (IRAKs) and the adaptor molecules TNF receptor-associated factors (TRAFs), and that lead to the activation of the mitogen-activated protein kinases (MAPKs) JUN N-terminal kinase (JNK) and p38, and to the activation of transcription factors. Two groups of transcription factors are activated downstream of TLR signaling; nuclear factor-κB (NF-κB) and interferon-regulatory factors (IRFs). This TLR signaling pathway leads to the induction of proinflammatory cytokines. Zinc influences this pathway on multiple levels. It augments MyD88-dependent signaling whereas inhibiting TRIF-mediated activation of IRF3/7. It has been shown that zinc inhibits IRAK thereby inhibiting further signal transductions. In vitro, zinc also directly impaired LPS-induced IKK activity.
Activation of Toll-like receptor (TLR) signaling pathways is mediated by a complex array of proteins. When TLRs bind ligands, dimerization of the ectodomain of TLRs is induced, bringing their cytoplasmic Toll/IL-1R domains together, resulting in the recruitment of intracellular adapter proteins and initiation of downstream signaling events. Following this, the Toll–IL-1-resistence (TIR) domains of TLRs engage TIR domain-containing adaptor proteins (either myeloid differentiation primary-response protein 88 (MYD88) or TIR domain-containing adaptor protein inducing IFNβ (TRIF) and TRIF-related adaptor molecule (TRAM)). This in turn stimulates downstream signaling pathways that involve interactions between IL-1R-associated kinases (IRAKs) and the adaptor molecules TNF receptor-associated factors (TRAFs), and that lead to the activation of the mitogen-activated protein kinases (MAPKs) JUN N-terminal kinase (JNK) and p38, and to the activation of transcription factors. Two groups of transcription factors are activated downstream of TLR signaling; nuclear factor-κB (NF-κB) and interferon-regulatory factors (IRFs). This TLR signaling pathway leads to the induction of proinflammatory cytokines. Zinc influences this pathway on multiple levels. It augments MyD88-dependent signaling whereas inhibiting TRIF-mediated activation of IRF3/7. It has been shown that zinc inhibits IRAK thereby inhibiting further signal transductions. In vitro, zinc also directly impaired LPS-induced IKK activity.
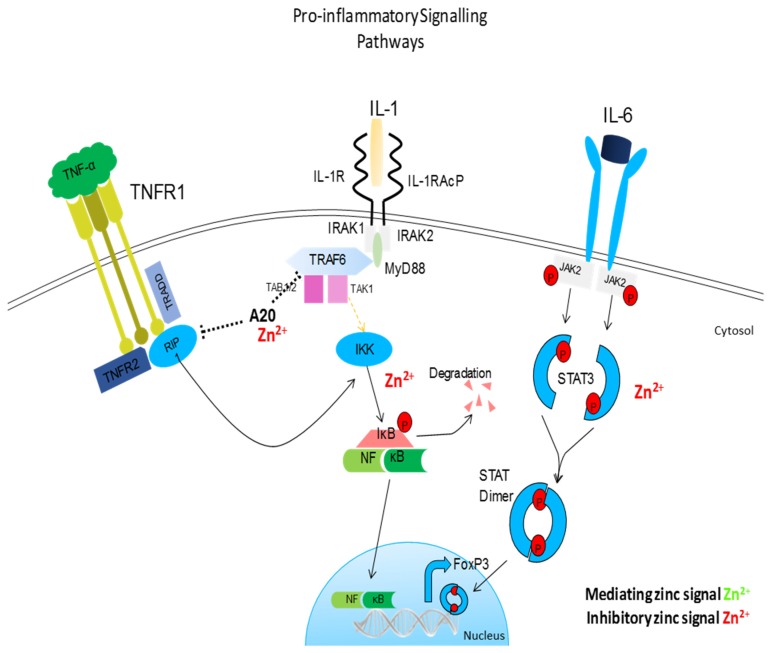 Pro-inflammatory signaling pathway influences by zinc. Similar to TLR signaling, IL-1, and TNF-R signaling pathways converge on a common IκB kinase complex that phosphorylates the NF-κB inhibitory protein, resulting in the release of NF-κB and its translocation to the nucleus. Zinc prevents the dissociation of NF-κB from its corresponding inhibitory protein, thus preventing the nuclear translocation of NF-κB and inhibiting subsequent inflammation. Zinc also inhibits IL-6-mediated activation of STAT3. Zinc acts as anti-inflammatory element influencing major pro-inflammatory signaling pathways.
Pro-inflammatory signaling pathway influences by zinc. Similar to TLR signaling, IL-1, and TNF-R signaling pathways converge on a common IκB kinase complex that phosphorylates the NF-κB inhibitory protein, resulting in the release of NF-κB and its translocation to the nucleus. Zinc prevents the dissociation of NF-κB from its corresponding inhibitory protein, thus preventing the nuclear translocation of NF-κB and inhibiting subsequent inflammation. Zinc also inhibits IL-6-mediated activation of STAT3. Zinc acts as anti-inflammatory element influencing major pro-inflammatory signaling pathways.
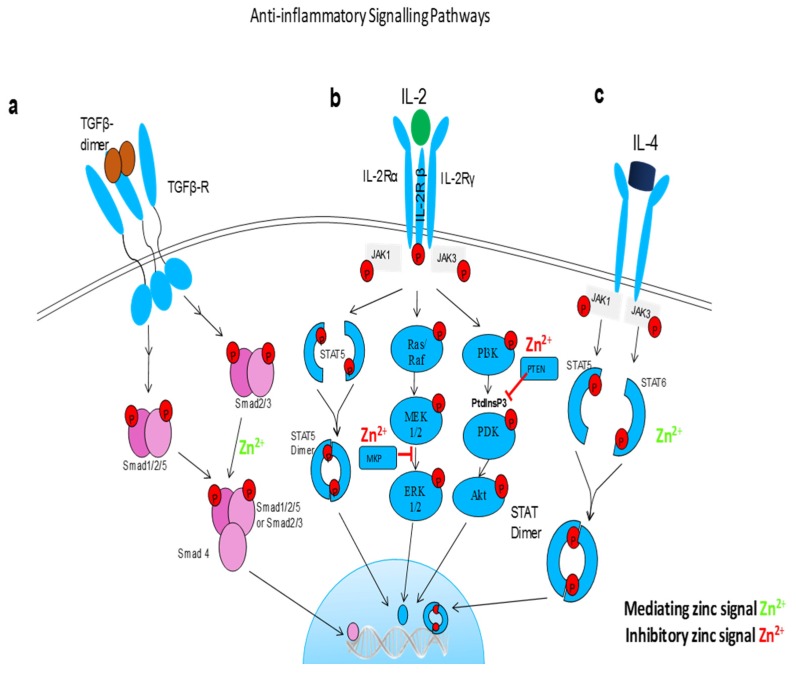 Anti-inflammatory signaling pathways influenced by free zinc. (a) TGFβ signaling is dependent on a dynamic on and off switch in Smad activity. Free zinc is a cofactor in Smad proteins and promote Smad 2/3 nuclear translocation and transcriptional activity. (b) zinc regulates IL-2 signaling pathway via blocking MAP kinase phosphatase (MKP) in extracellular signal-regulated kinases (ERK) 1/2 pathways and Phosphatase and tensin homologue (PTEN) which opposes phosphoinositide 3-kinase (PI3K) function in PI3k/Akt pathway. (c) free zinc phosphorylates STAT6 and promotes translocation of STAT dimers into the nucleus, hence promote the anti-inflammatory effects of Il-4.
Anti-inflammatory signaling pathways influenced by free zinc. (a) TGFβ signaling is dependent on a dynamic on and off switch in Smad activity. Free zinc is a cofactor in Smad proteins and promote Smad 2/3 nuclear translocation and transcriptional activity. (b) zinc regulates IL-2 signaling pathway via blocking MAP kinase phosphatase (MKP) in extracellular signal-regulated kinases (ERK) 1/2 pathways and Phosphatase and tensin homologue (PTEN) which opposes phosphoinositide 3-kinase (PI3K) function in PI3k/Akt pathway. (c) free zinc phosphorylates STAT6 and promotes translocation of STAT dimers into the nucleus, hence promote the anti-inflammatory effects of Il-4.| PMID: | 28629136 |
|---|---|
| PMCID (Free PMC Article): | PMC5490603 |
| DOI: | 10.3390/nu9060624 |
| Category: | Allergy / Immune |
The best supplements with Zinc in Allergy / Immune category:
- Zinc Picolinate, 100 Tablets (Solgar) - Zinc promotes healthy skin and supports normal taste and vision. It contains among others: Zinc.
- Immune Defence - Zinc Lozenges with Rosehip and Acerola - Your immune system needs daily support to stay in good form, to be able to fight off any pathogens trying to enter your system and to witstand the impact of stress on your body and mind. It contains among others: Zinc.
- Zinc Glycinate, 120 Softgels (Now Foods) - Zinc is a mineral co-factor in hundreds of enzymatic reactions related to protein and carbohydrate metabolism, RNA/DNA synthesis, and intercellular signaling. It contains among others: Zinc.
- L-OptiZinc, 30 mg, 100 Veg Capsules (Now Foods) - L-OptiZinc is a form of Zinc complexed with the amino acids Methionine. It contains among others: Zinc.
- Zinc, 50 mg, 250 Tablets (Now Foods) - Zinc is essential to the normal function of many organs and systems within the body including the skeletal, immune, neurological, and endocrine systems. It contains among others: Zinc.
- Zinc Picolinate, 50 mg, 120 Veg Capsules (Now Foods) - Zinc is essential to the normal function of many organs and systems within the body including the skeletal, immune, neurological, and endocrine systems. It contains among others: Zinc.
Previous article
Efficacy of Zinc Against Common Cold Viruses: An Overview.

























































